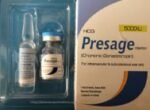

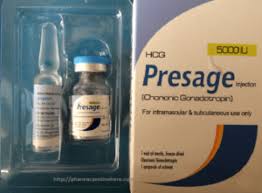
Hcg Presage Injection 5000lu
₨ 883
Out of stock
Pregnancy
Contraindicated in pregnancy. Lactation: Caution advised or effect undetermined. Precautions: Monitor hormone levels in anovulatory infertility. Asthma, epilepsy, migraine, cardiac or renal disorders.
Indications
In the Males: Hypogonadotropic hypogonadism, delayed puberty associated with insufficient gonadotrophic pituitary function, cryptorchidism (not due to anatomical obstruction). In the Females: Ovulation induction due to anovulation or impaired follicle-ripening and in controlled Hcg Presage Injection 5000lu hyperstimulation regimens to prepare the follicles for function
Dosage
Males: Hypogonadotropic hypogonadism: Adults: 1000-2000 IU 2-3 times per wk. If the main complaint is sterility (deficient spermatogenesis) in combination with menotrophin (75 IU FSH+75 IU HLH daily or 2-3 times a wk for at least 3 mth). Once achieved, the improvement in spermatogenesis may Hcg Presage Injection 5000lu in some cases be maintained by hCG alone. Delayed puberty associated with insufficient gonadotrophic pituitary function: Adults: 1500 IU 2-3 times a wk for at least 6 mth. Females: Ovulation induction due to anovulation or impaired follicle-ripening: Adults: One inj of 5000-1000 IU to complete treatment with a menotrophin preparation. Ovulation induction in controlled hyperstimulation regimens to prepare the follicles for function: Adults: Up to three repeat injections of up to 5000 IU may be given within the following 9 days to prevent insufficiency of the corpus luteum. Children: Prepubertal cryptorchidism: IM.4000 IU 3 times/wk or 5000 IU every sec day for 4 injections or 500 IU 3 times/wk for 4-6 wk or 15 injections of 500-1000 IU given over 6 wk. Hypogonadotropic hypogonadism: IM. 1 mth-18 yr, 1000-2000 IU twice wkly, adjusted to response.
Advice to Patient
If you are self-administering this medication, follow directions for reconstitution, injection and needle disposal. Administer exactly as directed. Contact prescriber if there is worsening of condition, swelling of legs/breasts/feet or if there is swelling/ redness/pain at injection site.
Administration
IM administration only. Stability: Following reconstitution with the provided diluent, solutions are stable for 30-90 days, depending on the specific preparation, when stored at 2 degree C to 15 degree C.
UNLICENSED USE
Children: Test of testicular function: IM. Short stimulation test: 1 mth-18 yr, 1500-2000 units once daily for 3 days. Prolonged stimulation test: 1 mth-18 yr, 1500-2000 units twice weekly for 3 wk.
Pharmacokinetics
Duration: IM: 36 hrs. Metabolism: Renal. Bioavailability: SC: 40%. Half-life elimination: Biphasic: First phase: 6-11 hrs. Terminal phase: 23-38 hrs. Time to peak: IM: 6 hrs. SC: 16-20 hrs. Excretion: Via urine within 24 hrs (10%-12% of dose).


Reviews
Clear filtersThere are no reviews yet.